Premarket Notification 510(k)
There are three types of Premarket Notification 510(k)s that are submitted to the FDA—Traditional, Special, and Abbreviated. The Special and Abbreviated methods were developed to streamline the 510(k) review process. Those two methods can only be used if certain criteria are met; however, the Traditional 510(k) method can be used in any situation.[1]
TRADITIONAL 510(K)
The important elements of a Traditional 510(k) include:
- Medical Device User Fee Cover Sheet (Form FDA 3601)
- CDRH Premarket Review Submission Cover Sheet
- Certification of Compliance with ClinicalTrials.gov Data Bank (FDA 3674)
- Cover letter as described in the format guidance
- Table of contents is recommended
- Indications for use
- 510(k) Summary (21 CFR 807.92) or 510(k) Statement (21 CFR 807.93)
- Standards Data Report for 510(k)s (FDA 3654) – This form is submitted if the 510(k) references a national or international standard.
- Truthful and Accuracy Statement (21 CFR 807.87(k))
- Class III Certification and Summary (21 CFR 807.94)—for Class III devices
- Information on sterilization, biocompatibility, expiration date, etc.—if applicable
- The information required for a Premarket Notification submission (items required under 21 CFR 807.87), includes: the name of the device (trade name or proprietary name and common or usual name or classification name); classification of the device; appropriate panel (ex. Cardiovascular, dental, etc.); product code (if known); description of the device (including device specifications and references to appropriate guidance documents, special controls, or standards); photographs or engineering drawings (if applicable); a comparison of the device with a predicate device(s) (focusing on similarities and differences, accompanied by data); information about the intended use of the device; and proposed labeling and advertisements for the device and directions for use.[2]
SPECIAL 510(K)
If a medical device that has been formerly cleared under the 510(k) process undergoes a modification, a Special 510(k) may be appropriate. The Special 510(k) “allows the manufacturer to declare conformance to design controls without providing the data.”[3] To incentivize medical device manufacturers to select this option for obtaining FDA clearance for device modifications, the Office of Device Evaluation (ODE) and the Office of In Vitro Diagnostic Device Evaluation and Safety (OIVD) intend to process Special 510(k)s within 30 days of receipt. The FDA is able to more efficiently review modifications that do not affect the device’s intended use or alter the device’s fundamental scientific technology.[4]
Examples of device modifications that are likely to be appropriate for review as Special 510(k)s include:
- A change in material formulation or change to a type of material that has been used in other legally marketed devices within the same classification regulation, for the same intended use
- Changes in an inactive or secondary ingredient or agent
- Energy type
- Environmental specifications
- Performance specifications
- Ergonomics of the patient/user interface
- Dimensional specifications
- Software or firmware
- Packaging or expiration dating
- Sterilization[5]
A comprehensive list of forms and elements required as part of a Special 510(k) is available here.
ABBREVIATED 510(K)
Medical device manufacturers may submit an Abbreviated 510(k) if or when a guidance document exists, a special control has been established, or the FDA has recognized a relevant consensus standard. With an Abbreviated 510(k) submission, medical device manufacturers “provide summary reports on the use of guidance documents and/or special controls, or declarations of conformity to recognized standards, to expedite the review of a submission.”[6]
An Abbreviated 510(k) submission must include the following elements:
- Items required in 21 CFR 807.87 (Information required in a Premarket Notification submission)
- Medical Device User Fee Cover Sheet (Form FDA 3601)
- CDRH Premarket Review Submission Cover Sheet
- Certification of Compliance with ClinicalTrials.gov Data Bank (FDA-3674)
- Cover Letter (as described in format guidance)
- Table of Contents (recommended)
- Indications for Use
- 510(k) Summary (21 CFR 807.92) or 510(k) Statement (21 CFR 807.93)
- Standards Data Report for 510(k)s (FDA 3654) (required if the 510(k) references a national or international standard)
- Truthful and Accuracy Statement (21 CFR 807.87(k))
- Class III Certification and Summary (for Class III devices) (21 CFR 807.94)
- For a submission that relies on a guidance document or special control(s), provide a summary report that describes how the guidance document or special control(s) were used to address the risks associated with the medical device
- For a submission that relies on a recognized standard, provide a Declaration of Conformity to a Recognized Standard
- Provide data to address issues not covered by guidance documents, special controls, or recognized standards
- Provide information on sterilization, biocompatibility, expiration date, etc.—if applicable[7]
More information about Abbreviated 510(k)s is available here.
510(K) APPROVAL PROCESS
Premarket Notification 510(k) submissions for medical devices are reviewed and approved by the Office of Device Evaluation (ODE) and the Office of In Vitro Diagnostics and Radiological Health (OIR)—both offices are within the FDA’s Center for Devices and Radiological Health (CDRH).
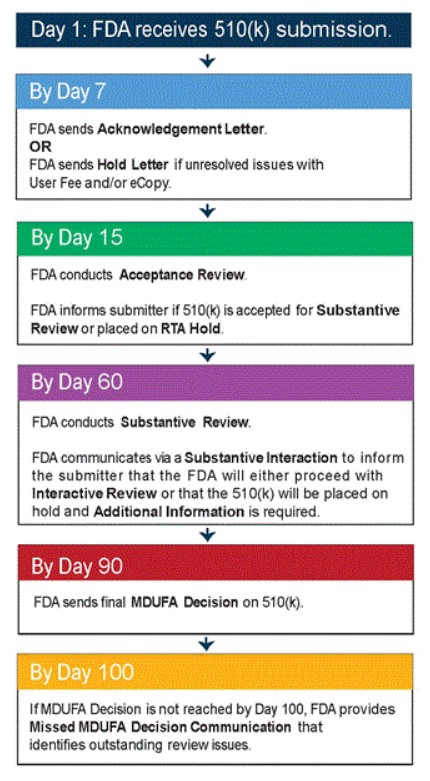
The process of submitting, reviewing, and approving a 510(k) submission takes approximately 90 days. Once the 510(k) submission has been sent to the FDA, the agency sends either an Acknowledgement Letter or a Hold Letter (if there are unresolved issues with eCopy or the user fee). Within approximately two weeks of submission, the FDA conducts an Acceptance Review. The agency informs the manufacturer if the 510(k) is accepted for Substantive Review or placed on RTA Hold. Within 60 days of submission, the FDA conducts the Substantive Review. The FDA communicates through a Substantive Interaction to inform the submitted that the agency will either proceed with an Interactive Review or that the 510(k) will be placed on hold (additional information will be required, if this is the case). Within 90 days of submission, the FDA will send a final MDUFA Decision on the 510(k). If a MDUFA (Medical Device User Fee Amendment) Decision is not reached within 100 days of submission, the FDA provides Missed MDUFA Decision Communication, which identifies any outstanding review issues.[8]
Source: FDA[9]
Updated by Kristin Stiner, November 2020

Comments are closed.