Office of Regulatory Affairs (ORA)
The Office of Regulatory Affairs (ORA) is charged with monitoring compliance of food and drug manufacturers and distributors. The office is the lead organization for all field operations and has regional offices around the country. ORA performs site visits to ensure regulatory compliance, as well as conducts criminal investigations in partnership with law enforcement agencies.[1] More specifically FDA is responsible for the following:
- Inspections of firms and plants producing FDA-regulated products
- Investigations of consumer complaints, emergencies and criminal activity
- Enforcement of FDA regulations
- Sample collection and analysis
- Review of imported products.[2]
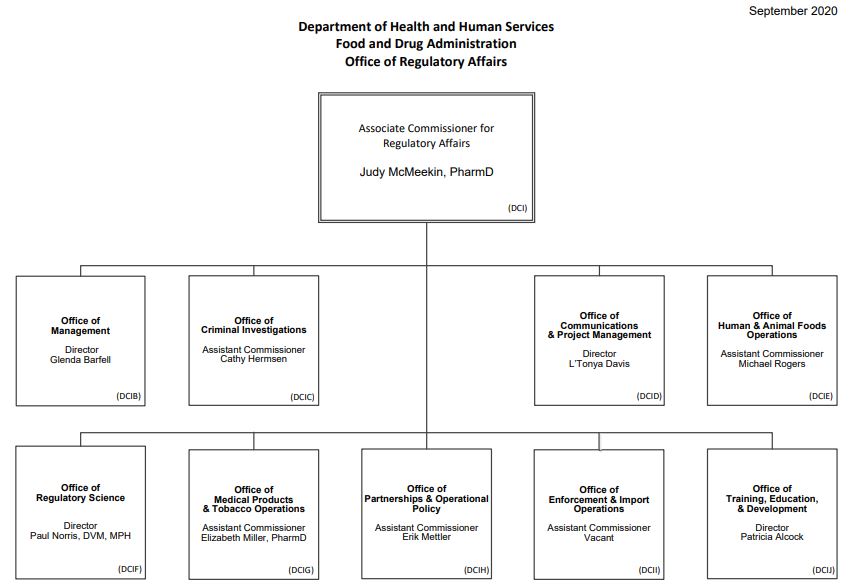
Source: ORA, 2020[3]
Small businesses that are interested in working with the FDA can find additional information and support on the FDA’s Small Business Assistance website.
ORA Small Business Representative
David Arvelo
FDA - Dallas District Office
1201 Main Street, Suite 7200
Dallas, Texas 75202
Phone: 214-253-4979

Comments are closed.