Center for Biologics Evaluation and Research (CBER)
The Center for Biologics Evaluation and Research (CBER) regulates biological and related products including blood, vaccines, allergenics, tissues, and cellular and gene therapies. CBER reviews new biologics and new indications for approved products. The center requires manufacturers to submit scientific and clinical data. CBER’s decision to approve a biologics product is based upon the risks and benefits of the product.[1]
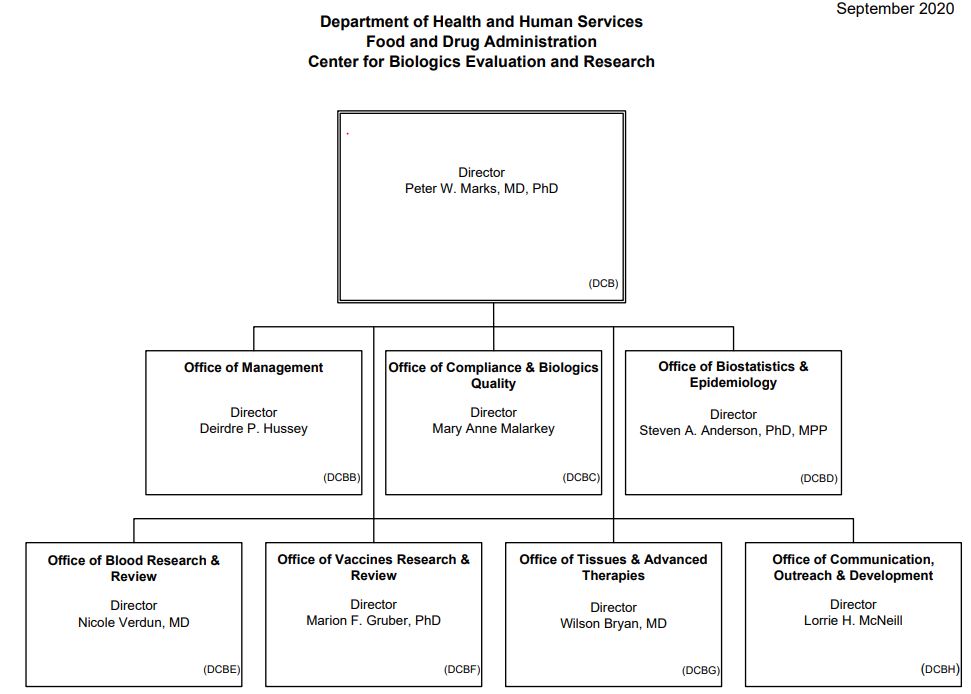
Source: CBER, 2020[2]
CBER has established a Manufacturers Assistance and Technical Training Branch to offer assistance and training to small and large manufacturers, as well as trade associations. The branch also facilitates requests for information pertaining to CBER policies and procedures. Industry assistance is offered in such areas as clinical investigator information, adverse event reporting procedures, electronic submissions guidance and requirements, and information on Investigational New Drug Application (IND) submission.[3]
Updated by Theresa Pipher, November 2020

Comments are closed.