Center for Veterinary Medicine (CVM)
The Center for Veterinary Medicine (CVM) is responsible for the regulation of the manufacture and distribution of animal feed, food additives, and drugs that are given to animals. This applies to animals raised for human consumption (livestock) and companion animals (pets), and includes drugs for minor species such as fish, hamsters, and parrots, as well as for minor uses in major species such as cattle, turkeys, and dogs.[1] For more on what CVM regulates, visit the following:
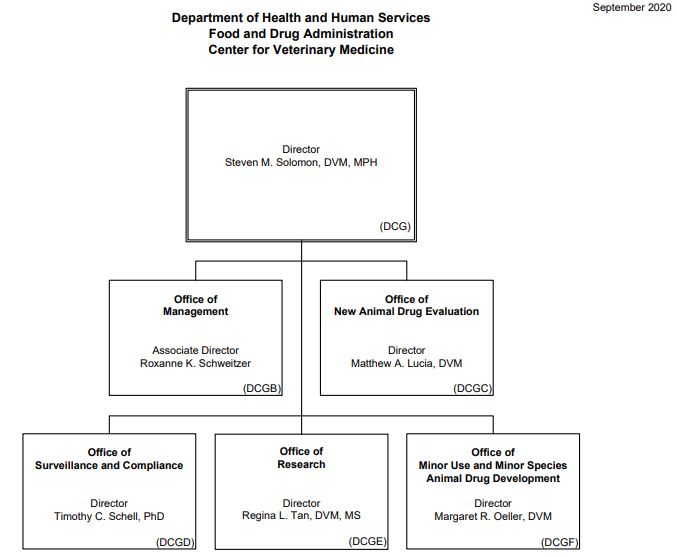
Source: CVM, 2020[2]
CVM develops and publishes guidance documents to assist industry in understanding the FDA’s current reasoning on various topics.[3] CVM provides an outline for new requirements, as well as those under development for industry. Some guidance documents are under development with drafts and/or finals expected to be released by the end of 2018.[4] One can also view the Center for Veterinary Medicine Strategic Human Capital Plan Fiscal Years 2017-2021, online.
Updated by Theresa Pipher, November 2020

Comments are closed.