Center for Drug Evaluation and Research (CDER)
The Center for Drug Evaluation and Research (CDER) regulates non-prescription (over-the-counter), generic drugs, and prescription drugs for both safety and efficacy before they are marketed. CDER also regulates and evaluates drug-like products such as fluoride toothpaste, antiperspirants, dandruff shampoos, and sunscreens. CDER approves alternate uses for approved drugs and ensures that the marketing of drugs to consumers complies with FDA guidelines and regulations. [2]
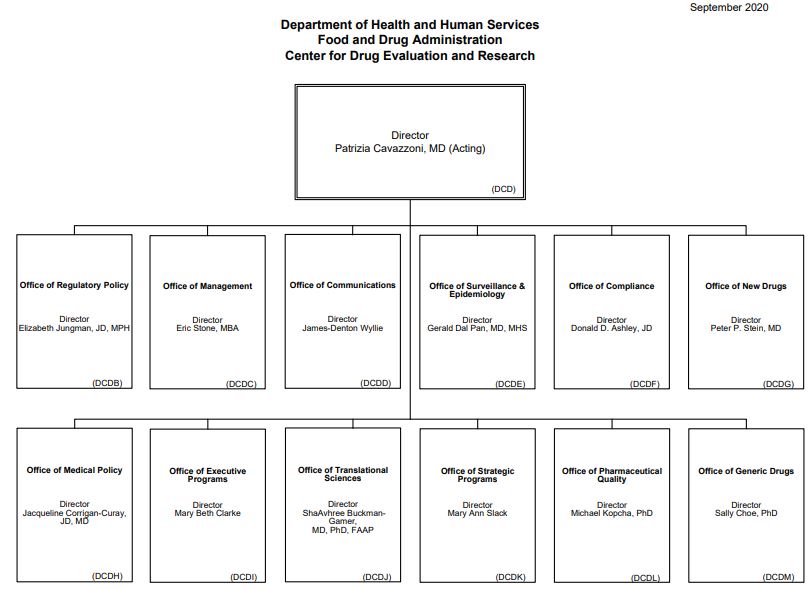
Source: CDER, 2020[3]
CDER provides advice to small businesses on such subjects as drug development, generic drug and OTC drug reviews, biosimilars, drug safety, and more through their Small Business & Industry Assistance (SBIA) program.[4] CDER SBIA offers a variety of online resources, via the following topic links:
- SBIA Multimedia Resources and Offerings
- Over-the-Counter Drug Regulation
- New Drug Development and Safety
- Regulatory Submissions
- Generic Drugs
- Registration and Listing
- Drug Supply Chain
- Import-Export
- Drug Quality
- BioResearch Monitoring Program, Drug Master File, Compounding, Drug Shortages
- Related Presentations and Webinars

Comments are closed.