INVESTIGATIONAL NEW DRUG (IND) APPLICATION
The primary purpose of the Investigational New Drug (IND) application is to supply data to the FDA depicting that it is reasonable to start human testing for a new drug. The IND application also allows sponsors to obtain an exemption from the FDA when distributing investigational drugs to clinical investigators across state lines, as current law requires drugs to have an approved marketing application prior to shipment.[1] [2]
Types of INDS
There are three types of INDs:
- Investigator IND: This type of IND is submitted by a physician who initiates and conducts the investigation, and who oversees the administration or dispensing of the investigational drug.
- Emergency Use IND: This type of IND enables the FDA to authorize the use of the experimental drug in emergency situations.
- Treatment IND: This type of IND is intended for experimental drugs that demonstrate promise in clinical testing for serious or near-term life-threatening conditions.[1]
Categories of INDS
The IND application, which can either be categorized as commercial (submitted by companies to obtain marketing approval for a new product) or research (non-commercial), contains sufficient information to allow an unapproved drug or biologic to be safely administered to humans. It must contain information pertaining to the three areas of: Animal Pharmacology and Toxicology Studies, Manufacturing Information, and Clinical Protocols and Investigator Information.[1]
Phases of an Investigation
An IND may be submitted for one or more phases of an investigation. The three phases of a clinical investigation for a previously untested drug are generally conducted in sequential order, but in some cases they may overlap. Phase 1 encompasses the initial introduction of the investigational new drug to humans. Typically conducted in healthy volunteers, these studies are used to determine metabolic and pharmacological actions of the drug in humans, determine potential side effects, and gauge effectiveness. Phase 2 is defined by early controlled clinical studies that are conducted in order to obtain preliminary data on the effectiveness of the drug for a specific indication(s) in patients with the identified disease or medical condition. Phase 3 studies are used to gather additional information related to drug effectiveness and safety—which is needed to evaluate the overall benefit/risk relationship of the drug.[2]
Resources for IND Applications
The following materials reflect resources designed to assist sponsors in the IND process; they include references to assistance programs, hyperlinked guidance documents, CFR regulations and additional relevant FDA provided topic links.
Pre-IND Consultation Program
The FDA’s Center for Drug Evaluation and Research (CDER) offers a Pre-Investigational New Drug (IND) Consultation Program to foster early communications between sponsors and new drug review divisions in order to provide guidance on the data necessary to warrant IND submission. The review divisions are organized generally along therapeutic class.
Guidance Documents for INDS
Guidance documents represent the FDA's current thinking on a particular subject. These guidance documents provide FDA review staff and applicants/sponsors with guidelines to the processing, content, and evaluation/approval of applications and also to the design, production, manufacturing, and testing of regulated products. Additionally, they establish policies intended to achieve consistency in the Agency's regulatory approach and establish inspection and enforcement procedures. However, these guidances are not regulations or laws, they are not enforceable, either through administrative actions or through the courts. An alternative approach, they may be used if it satisfies the requirements of the applicable statute, regulations, or both. For information on a specific guidance document, one may contact the originating office, and to find guidance documents to help prepare INDs, go to Guidances (Drugs) and use "investigational" in the search box. [3]
For more guidance related to drugs:
- Go to Search for FDA Guidance Documents
- Scroll down to Guidance Document Search
- Filter by FDA Organization, Center for Drug Evaluation and Research
- Use other filters, as needed, including those noted below.[4]
Laws, Regulations, Policies and Procedures
The Federal Food, Drug, and Cosmetic Act is the basic food and drug law for the United States. This law assures consumers that drugs and devices are both safe and effective for their intended uses.
The Code of Federal Regulations is divided into 50 titles that represent areas subject to Federal regulation. Section 21 of the CFR contains most of the regulations pertaining to food and drugs. The following regulations are applicable to the IND application process:
- 21CFR Part 312: Investigational New Drug Application
- 21CFR Part 314: INDA and NDA Applications for FDA Approval to Market a New Drug (New Drug Approval)
- 21CFR Part 316: Orphan Drugs
- 21CFR Part 58: Good Lab Practice for Nonclinical Laboratory [Animal] Studies
- 21CFR Part 50: Protection of Human Subjects
- 21CFR Part 56: Institutional Review Boards
- 21CFR Part 201: Drug Labeling
- 21CFR Part 54: Financial Disclosure by Clinical Investigators
The document titled Expanded Access to Investigational Drugs for Treatment Use – Questions and Answers (2016) is a guidance document pertaining to the emergency use of an investigational drug or biologic.
Additional IND Resources
The FDA provides additional resources related to the IND application process—these resources are available here.
IND Review Process
The following graphic depicts the IND review process.
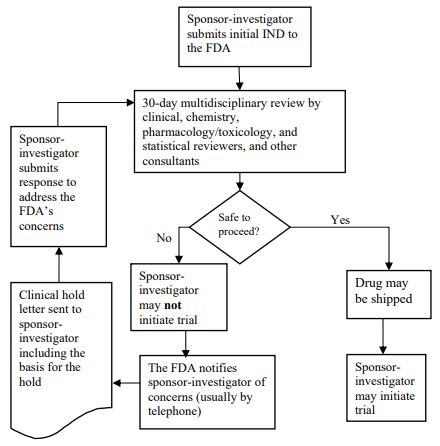
Source: FDA, 2015[5]
Updated by Theresa Pipher, November 2020


Comments are closed.