Center for Devices and Radiological Health (CDRH)
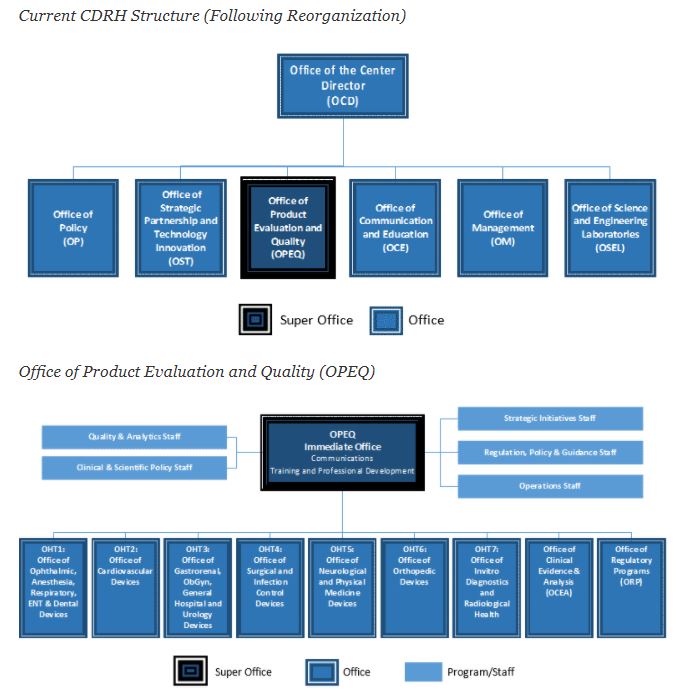
In 2019, the FDA's Center for Devices and Radiological Health's (CDRH) reorganized, creating an agile infrastructure that can adapt to future organizational, regulatory and scientific needs. The reorganization did not change the mission of CDRH. FDA sources indicate that the CDRH reorganization is designed to improve organizational efficiencies to better meet public health needs, noting that CDRH's premarket and postmarket program functions will be integrated along product lines. The current CDRH structure, following reorganization, follows:
Org image source
The following bullets capture the CDRH management directory, by organization:
The CDRH offers assistance and information to small businesses and other medical device manufacturers here. This includes online courses on the safety and effectiveness of medical devices and exposure to radiation from medical devices; a list of upcoming workshops and conferences dealing with FDA approval of medical devices; as well as facilitating the Medical Device Fellowship Program, where health professionals can participate in the FDA regulatory process for medical devices. Additional resources available through the CDRH for industry include the Device Advice page on product and regulatory information, bringing a device to market, a searchable medical device database, and COVID 19 resources.[3]

Comments are closed.